

The median age was 78 years and the median creatinine clearance was 52.6 mL/min. Approximately half of the patients in each group were male. RE-VERSE AD included data for 503 patients: 301 patients with serious bleeding (Group A) and 202 patients requiring an urgent procedure/surgery (Group B).

A key secondary endpoint was the restoration of haemostasis. The primary endpoint was the maximum percentage reversal of the anticoagulant effect of dabigatran within 4 hours after the administration of idarucizumab, based on central laboratory determination of dilute thrombin time (dTT) or ecarin clotting time (ECT). One prospective, open-label, non-randomized, uncontrolled study (RE-VERSE AD) was conducted to investigate the treatment of adult patients who presented with dabigatran-related life-threatening or uncontrolled bleeding (Group A) or who required emergency surgery or urgent procedures (Group B). Representative values for pharmacokinetic and pharmacodynamics parameters were established on the basis of healthy subjects aged 45-64 years receiving 5 g idarucizumab (see sections 5.1 and 5.2). In these studies the doses of idarucizumab ranged from 20 mg to 8 g and the infusion times ranged from 5 minutes to 1 hour. The investigated population consisted of healthy subjects and subjects exhibiting specific population characteristics covering age, body weight, race, sex and renal impairment.
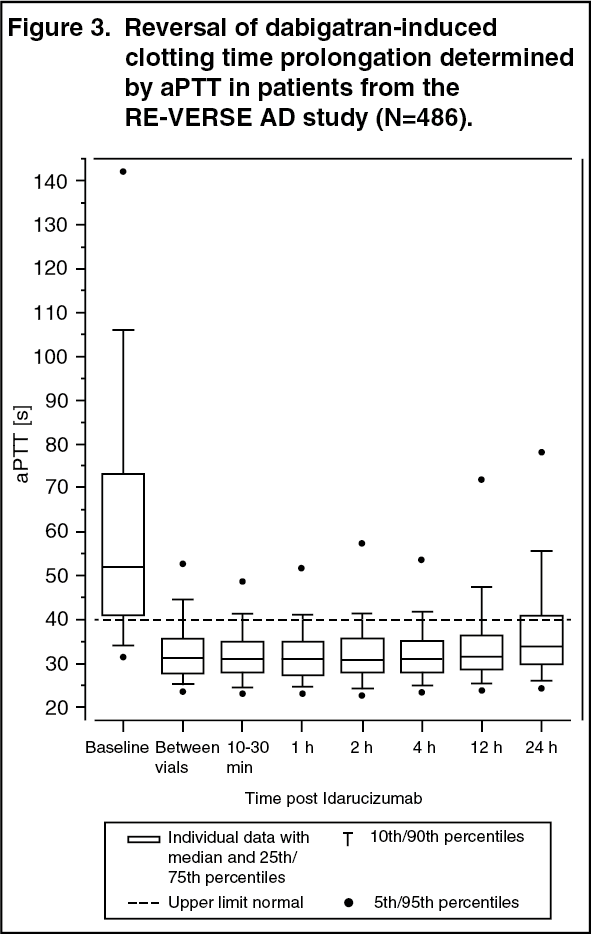
Three randomised, double-blind, placebo-controlled Phase I studies in 283 subjects (224 treated with idarucizumab) were conducted to assess the safety, efficacy, tolerability, pharmacokinetics and pharmacodynamics of idarucizumab, given alone or after administration of dabigatran etexilate. Idarucizumab potently and specifically binds to dabigatran and its metabolites and neutralises their anticoagulant effect. The idarucizumab-dabigatran complex is characterised by a rapid on-rate and extremely slow off-rate resulting in a very stable complex. It is a humanized monoclonal antibody fragment (Fab) that binds to dabigatran with very high affinity, approximately 300-fold more potent than the binding affinity of dabigatran for thrombin. Idarucizumab is a specific reversal agent for dabigatran. Pharmacotherapeutic group: all other therapeutic products, antidotes ATC code: V03AB37 Mechanism of action


 0 kommentar(er)
0 kommentar(er)
